On March 25th, a research paper entitled “Centrosome Anchoring Regulates Progenitor Properties and Cortical formation” was published online in Nature by the groups of Dr. Song-Hai Shi and Dr. Hang Shi in the School of Life Sciences, IDG/McGovern Institute for Brain Research and Advanced Innovation Center for Structural Biology, Tsinghua University. This study, for the first time, revealed a crucial role of the centrosome in regulating the mechanical properties and proliferation of neural progenitor cells, and consequently the size and configuration of the mammalian cerebral cortex.
Radial glial cells are the major neural progenitor cells in the developing mammalian cerebral cortex responsible for producing essentially all cortical neurons as well as glial cells. Distinct from other mammalian cells that harbor the centrosome next to the nucleus, radial glial progenitors (RGPs) position their centrosomes away for the nucleus at the luminal surface of the ventricular zone. The molecular and cellular nature and the precise function of this highly unique and characteristic subcellular organization of the centrosome in RGPs have been a mystery since the initial observation decades ago.
In this study, the authors performed serial section transmission electron microscopy analysis and revealed that the centrosome of mouse cortical RGPs is anchored at the apical membrane via the distal appendages (DAPs) preferentially assembled at the mother centriole (Figure 1). Selective removal of CEP83, a DAP-specific protein, in cortical RGPs eliminates DAPs and prevents the centrosome from docking to the apical membrane, resulting in a subtle (<1 mm) dislocation of the centrosome away from the apical membrane. Remarkably, this small centrosomal misposition impairs the specific formation of ring-like microtubule structure at the apical membrane and causes apical membrane stretching and stiffening, resulting in excessive activation of mechanically-sensitive Yes-associated protein (YAP) in RGPs. This over-activation of YAP leads to a drastic increase in an initial RGP proliferation (i.e., lateral expansion) and subsequent intermediate progenitor production (i.e., radial expansion), and the formation of a substantially enlarged cortex with abnormal folding.
This study resolves the long-lasting mystery of the function and mechanism of the unique centrosomal subcellular organization in RGPs. It adds an entirely new dimension to the regulation of RGP behavior and cortical development. In addition, while mutations of the centrosomal proteins have been strongly associated with microcephaly (i.e., small brain), this study, for the first time, demonstrates that mutation in the centrosomal protein can also lead to megalencephaly (i.e., large brain). More importantly, human patients with biallelic CEP83 mutations exhibit enlarged brain ventricles and intellectual disability, in addition to infantile nephronophthisis. Therefore, this study also provides important new insights into human cortical malformation and intellectual disability.
Ph.D. student Wei Shao from Weill Cornell Medical College and Ph.D. student Jiajun Yang from the School of Life Sciences at Tsinghua University are the co-first authors of the study. Ph.D. students Ming He from Peking University, Xiang-Yu Yu from Tsinghua University, Zhaohui Yang from Weill Cornell Medical College, Alexandra L. Joyner, Kathryn V. Anderson and Meng-Fu Bryan Tsou from the Memorial Sloan Kettering Cancer Centre, Choong Heon Lee and Jiangyang Zhang from New York University also contribute to the study. This study is supported by Tsinghua–Peking Joint Centre for Life Sciences, Beijing Advanced Innovation Center for Structural Biology, Beijing Frontier Research Center for Biological Structure, Beijing Outstanding Young Scientist Program, NIH and Howard Hughes Medical Institute.
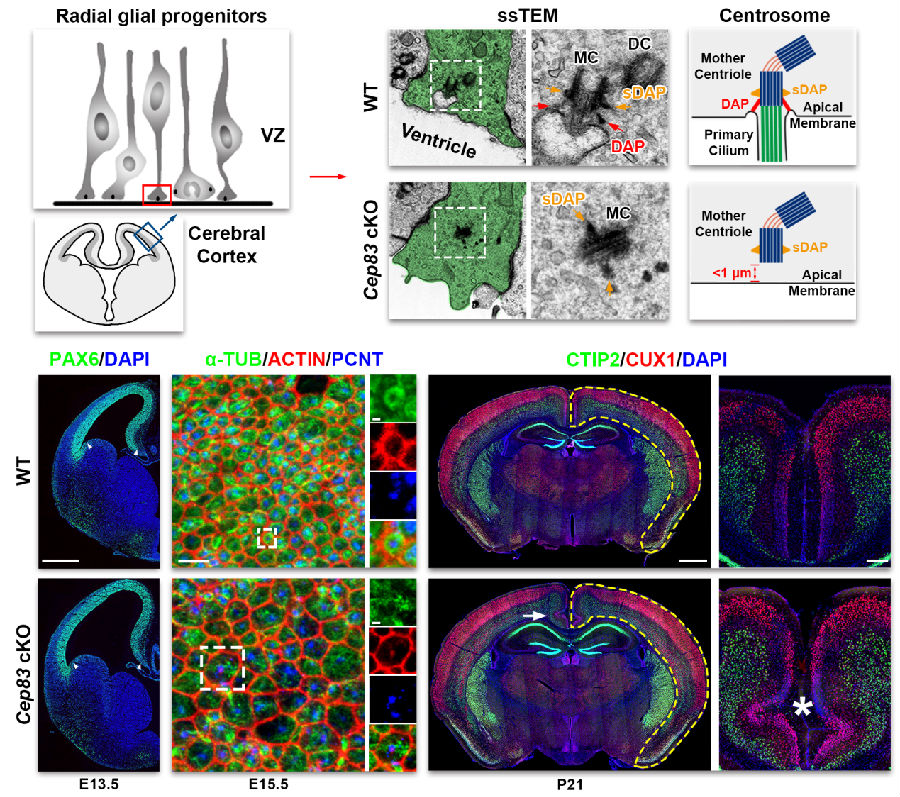
Figure 1. Centrosome apical membrane anchoring regulates the mechanical properties of RGPs and cortical size and configuration.
Paper link: https://www.nature.com/articles/s41586-020-2139-6
