A joint team led by Prof. Wei Xie at Tsinghua University and Prof. Antoine H.F.M. Peters from Friedrich Miescher Institute for Biomedical Research has published a research paper in Molecular Cell on December 11th, entitled “Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos”. This study reveals a unique chromatin organization, Polycomb Associating Domain (PAD), in mouse late stage oocytes and illustrates the critical role of Polycomb proteins in regulating PADs.
In eukaryotes, the linear DNA is compactly packaged in the nucleus through hierarchical organization. The proper folding of chromatin fiber is crucial for fundamental cell processes including gene expression and cell division. Mammalian oogenesis is accompanied with drastic chromatin reorganization. For example, late stage oocytes gradually transit from transcriptionally active state into the transcriptionally silenced state with highly condensed chromatin structure. Because of the limitation of cell number and technology, a comprehensive study of chromatin architecture re-folding during mammalian oogenesis is still lacking. To address the issue, this study applied low-input Hi-C method (sisHi-C) to systematically examine the reprogramming of chromatin organization during oogenesis and early embryonic development in mice. They found that primordial germ cells (PGCs) have relatively classical chromatin structure with clear TADs and compartmental patterns. However, during follicle growth, full-grown oocytes (FGOs) show distinct non-canonical chromatin configuration with the depletion of conventional distal plaid patterns (compartments). Instead, a new compartmental pattern restricted to local regions can be detected. Intriguingly, these self-interacting compartmental domains match well with H3K27me3 marked regions in FGOs, therefore termed Polycomb Associating Domains (PADs). Meanwhile the interspaced regions are named after iPADs (inter PADs). PADs/iPADs disassemble upon meiotic resumption from diplotene arrest but briefly re-appear on the maternal genome after fertilization. Upon maternal depletion of Eed, core components of Polycomb Repressive Complex 2 (PRC2), PADs are largely intact in oocytes, but their re-establishment after fertilization is compromised. By contrast, the depletion of Polycomb Repressive Complex 1 (PRC1) proteins attenuates PADs in oocytes. In addition, cohesin is dispensable for PADs in mouse oocytes, as PADs are not weakened in Scc1, an essential component of Cohesin, conditionally knocked out oocytes. Finally, investigators indicate that PADs may play a role in gene repression in FGOs. Taken together, these data reveal a unique chromatin configuration “PAD” in mouse oocytes and the critical role of Polycomb proteins in regulating chromatin architecture during mammalian oocyte growth and early development.
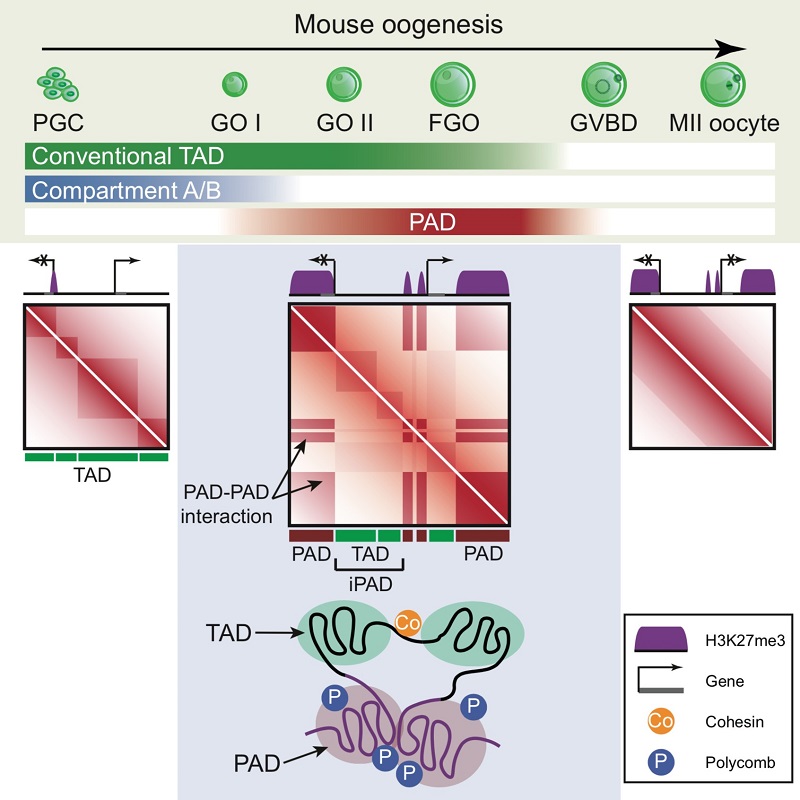
Figure 1. A schematic model showing the dynamics of chromatin organization during mouse oogenesis.
Prof. Wei Xie from School of Life Sciences of Tsinghua University and Prof. Antoine H.F.M. Peters from Friedrich Miescher Institute for Biomedical Research are the co-corresponding authors of this work. Postdoc fellow Zhenhai Du, Hui Zheng from CLS program of School of Life Sciences at Tsinghua University, Yumiko K. Kawamura from Friedrich Miescher Institute for Biomedical Research and Ph.D candidate Ke Zhang from School of Life Sciences at Tsinghua University are the co-first authors of this work. Johanna Gassler, Sean Powell from Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Evgeniy A. Ozonov, Nathalie Véron from Friedrich Miescher Institute for Biomedical Research, Postdoc fellow Qianhua Xu, Zili Lin from CLS program of School of Life Sciences at Tsinghua University, Ph.D candidates Kai Xu, Lijia Li, Guang Yu from School of Life Sciences at Tsinghua University and Qian Zhou from Institute of Zoology also make great efforts in this work. Collaborators include Kikue Tachibana’s group from Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Hiroyuki Sasaki ’s group from Medical Institute of Bioregulation at Kyushu University, Qingyuan Sun’s group from Institute of Zoology, Jie Na ’s group from School of Medicine at Tsinghua University, Yujie Sun’s group from Biodynamic Optical Imaging Center at Peking University, Aibin He’s group from Institute of Molecular Medicine at Peking University , the animal facility and the sequencing facility at Tsinghua University. This work is supported by the funding provided by the National Key R&D Program of China, the National Natural Science Foundation of China, the National Basic Research Program of China, Beijing Municipal Science & Technology Commission, the THU-PKU Center for Life Sciences, HHMI International Research Scholar, the Novartis Research Foundation, the European Research Counsel and JSPS KAKENHI.
Paperlink: https://www.cell.com/molecular-cell/fulltext/S1097-2765(19)30840-8#
